Mission
Motus GI® provides innovative solutions to address unmet needs in GI endoscopy, cost-effectively improving clinical outcomes that benefit patients, providers and payers worldwide.
Core Values
We Value Our Team and Their Diversity.
We Are Committed to Improving Patients’ Lives.
We Instil Care and Quality in All We Do.
We Persevere to Deliver All of Our Commitments.
Company Overview
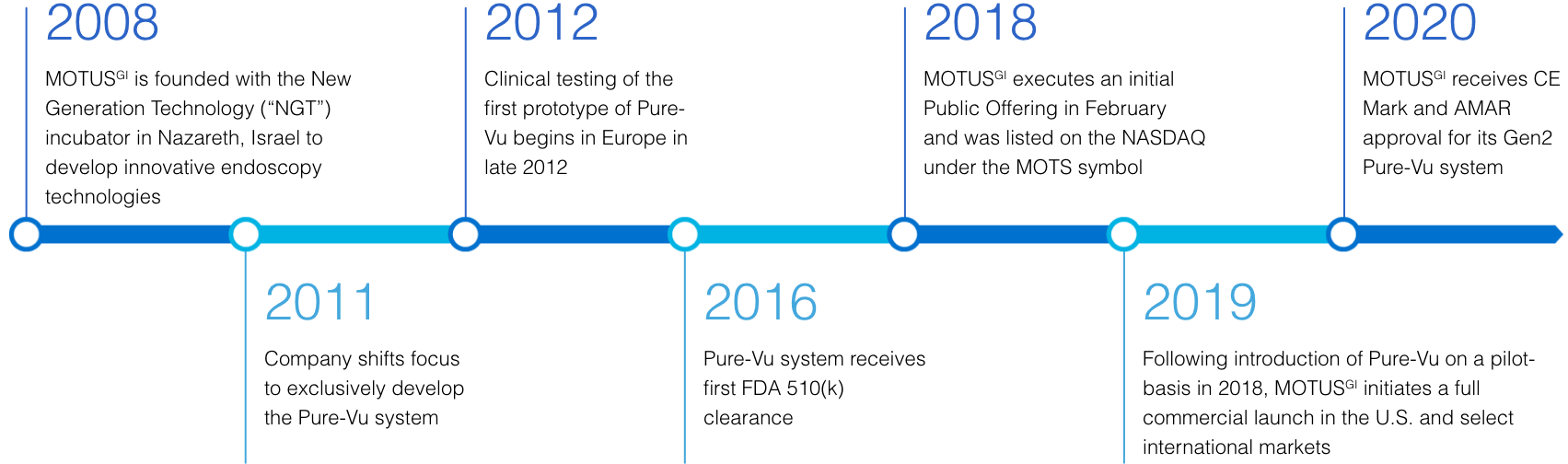
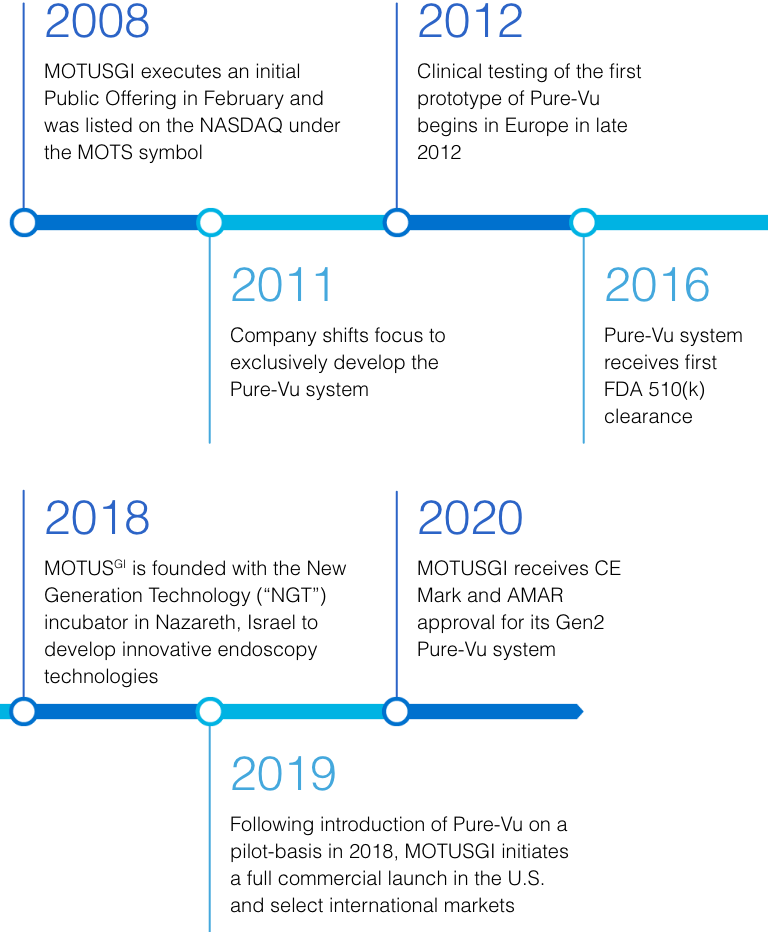
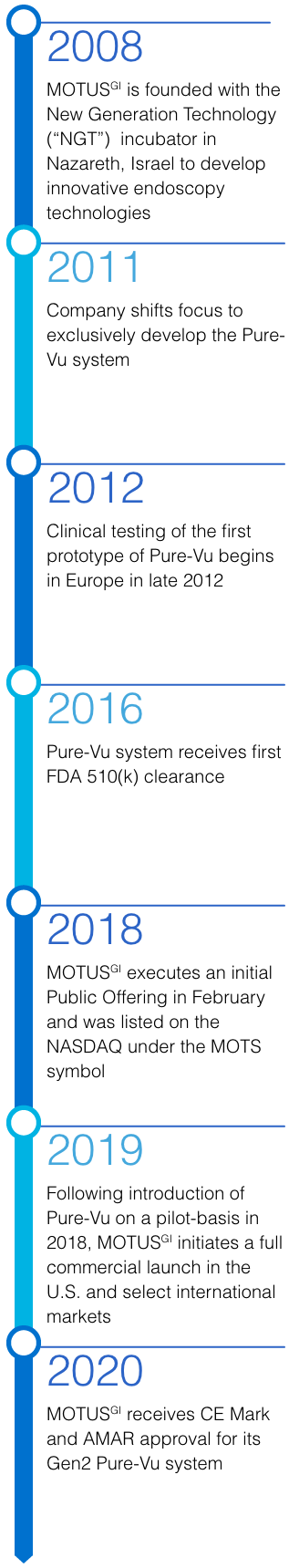
Motus GI Holdings, Inc. is a medical technology company, with subsidiaries in the U.S. and Israel, dedicated to improving clinical outcomes and enhancing the cost-efficiency of colonoscopy. The Company’s flagship product is the Pure-Vu® System, a U.S. FDA cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes to enable safe and rapid cleansing during the procedure while preserving established procedural workflow and techniques. The first generation of the Pure-Vu® System has received CE mark approval in Europe. The Pure-Vu® System is currently being introduced on a pilot basis in the U.S. market, and the Company is planning to initiate a commercial launch focused on the U.S. hospital market in 2019.
Challenges with bowel preparation for inpatient colonoscopy represent a significant area of unmet need that directly affects clinical outcomes and increases the cost of care in a market segment that comprises approximately 1.5 million annual procedures in the U.S. and approximately 4 million annual procedures worldwide. Motus GI believes the Pure-Vu® System may improve outcomes and lower costs for hospitals by reducing the time to successful colonoscopy, minimizing delayed and incomplete procedures, and improving the quality of an exam. In clinical studies to date, the Pure-Vu® System significantly increased the number of patients with an adequate cleansing level, according to the Boston Bowel Preparation Scale Score, a validated assessment instrument.