Clinical Evidence
Outstanding Cleansing Performance
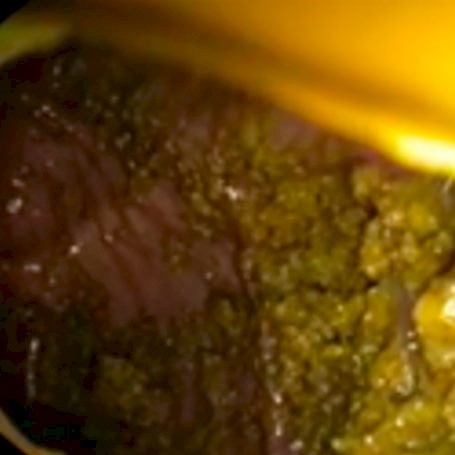
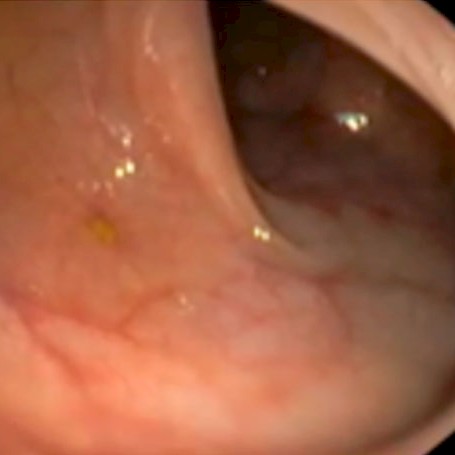
In clinical studies, the Pure-Vu® System was demonstrated to be safe and effective in cleaning inadequately prepared colons to an adequate level for a thorough exam.
In four clinical research studies including more than 250 patients, the Pure-Vu® system demonstrated effective cleansing performance, with statistically significant improvements in Boston Bowel Prep Scale (BBPS) ratings.
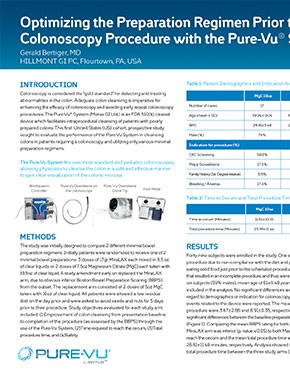
Before Pure-Vu
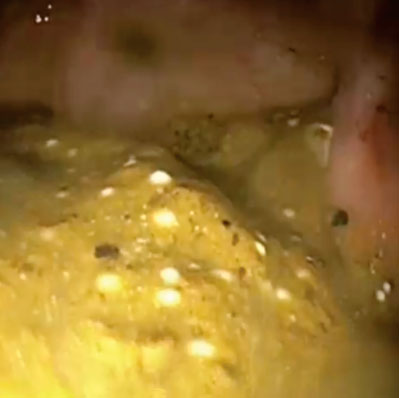
After Pure-Vu
Adequate Bowel Preparation Rate Improved from 38% to 96% Following Use of Pure-Vu® System1
| Mean BBPS | Pre‑PURE‑ VU Use |
Post‑PURE‑ VU Use |
P Value <0.001 |
|---|---|---|---|
| Descending Colon, Sigmoid and Rectum | 1.74 | 2.89 | <0.001 |
| Transverse Colon | 1.74 | 2.91 | <0.001 |
| Ascending and Cecum | 1.50 | 2.86 | <0.001 |



Similar cleansing results have been found in other clinical studies involving 150+ patients
Multi-center Study 1 and 2
BBPS Rating Before and After the
Use of the Pure-Vu® System




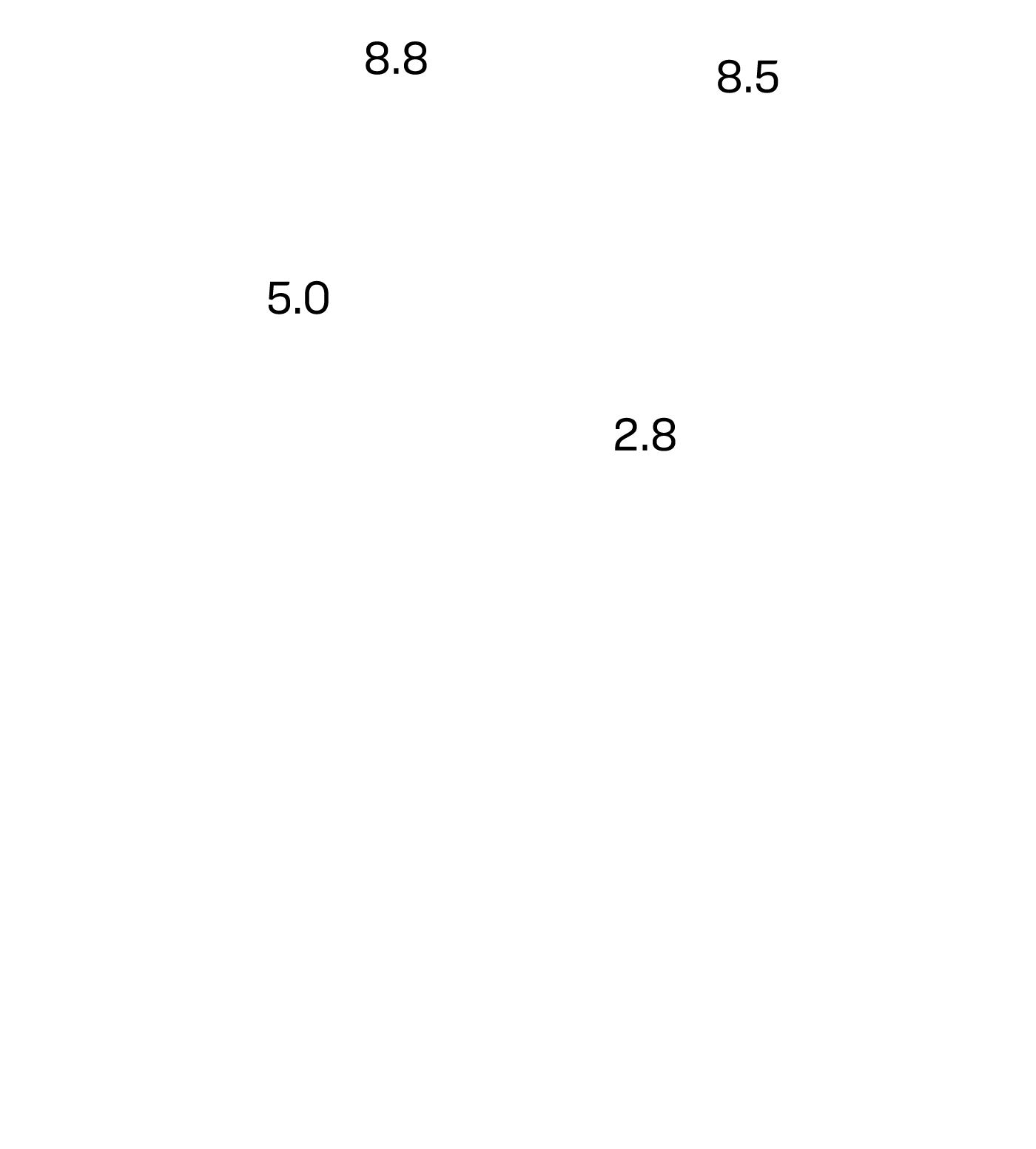




Before Pure-Vu
After Pure-Vu
Before Pure-Vu

After Pure-Vu

Patients were given a pre-procedural preparation consisting of only an 18-24 hour liquid diet and 4 bisacodyl/laxadin tablets (Dulcolax) to purposely create inadequately prepped colons. No traditional purgative was given. The Pure-Vu® System significantly increased the number of subjects with an adequate cleansing level (BBPS ≥ 2 in each colon segment) from 31% at baseline to 98% after use of the Pure-Vu® System (p<0.001). Physicians were satisfied with the device’s general ease of use. No major difficulties were experienced when performing polypectomy. No serious adverse events were reported.
Single-center Study 3









Patients were randomized to receive one of two minimal bowel preparations: three doses of 17gr MiraLAX each mixed in 8.5 oz of clear liquids or two doses of 7.5oz Magnesium Citrate (MgC) each taken with 19.5oz of clear liquid. A study amendment early on replaced the MiraLAX arm, due to obvious inferior Boston Bowel Preparation Scale (“BBPS”), a validated assessment instrument, scoring from the outset. The replacement arm consisted of two doses of 5oz MgC taken with 16oz of clear liquid. All patients were allowed to eat a low residue diet (consisting of chicken, pasta, eggs, etc.) on the day prior and were asked to avoid seeds and nuts for five days prior to their procedure. Study objectives evaluated for each study arm included: (1) improvement of colon cleansing from presentation baseline to completion of the procedure (as assessed by the BBPS) through the use of the Pure-Vu® System, (2) time required to reach the cecum, (3) total procedure time, and (4) safety.
No significant differences were found between the three groups with regard to demographics or indication for colonoscopy. No serious adverse events related to the device were reported. The use of the Pure-Vu® System enabled successful intraprocedural cleansing of the colon and ensured successful completion of all colonoscopies performed (100% success rate). Patients in the study had an average baseline BBPS of 3.67±2.86 which was improved to an average of 8.91±0.35 (p value p<0.0001) following use of the Pure-Vu® System.
The Pure-Vu® System was found to be safe, efficacious, and easy to use in cleansing inadequately prepared colons, enabling the endoscopist to conduct a complete examination. The use of the Pure-Vu® System added some time to the procedure, but the total procedure time was approximately 25 minutes in this study.