Frequently Asked
Questions
FAQs
Explore our frequently asked questions (FAQs) for answers to common inquiries, such as product availability, technically capabilities, service, and technical support.
Don’t see what you’re looking for? Email us at marketing@motusgi.com. Let us know if there’s a topic or product that you’d like further information about.
Product Availability
Is the Pure-Vu® system available for purchase?
Yes. The Pure-Vu® System is commercially available in various regions of the United States. With a commitment to provide dedicated support to customers as they integrate the System into their facilities, MOTUSGI is currently limiting its focus and resources to select geographic regions.
Is the Pure-Vu® System available outside of the United States?
The Pure-Vu® System received CE Mark in February 2018; however, MOTUSGI efforts are currently focused on commercialization in the United States. Commercial activities in Europe are expected to start in 2020/2021.
Are you currently using distributors to commercialize the Pure-Vu® System?
MOTUSGI is currently utilizing a direct sales force for the Pure-Vu® System commercialization efforts in the United States.
Capabilities & Clinical Evidence
Is the Pure-Vu® System for both inpatients and outpatients?
Yes. Pure-Vu® can be used for both inpatient and outpatient settings.
What facilities does MOTUSGI currently work with?
A number of major medical centers across the United States are currently using the Pure-Vu® System to clean poorly-prepped colons. For more information, please contact a MOTUSGI representative.
Is MOTUSGI looking for clinical trial sites?
At present, MOTUSGI is not actively looking for additional clinical trial sites. However, if you have interest in participating in an upcoming clinical trial, please contact a MOTUSGI representative. The clinical team assesses clinical needs and identifies appropriate partners for each study.
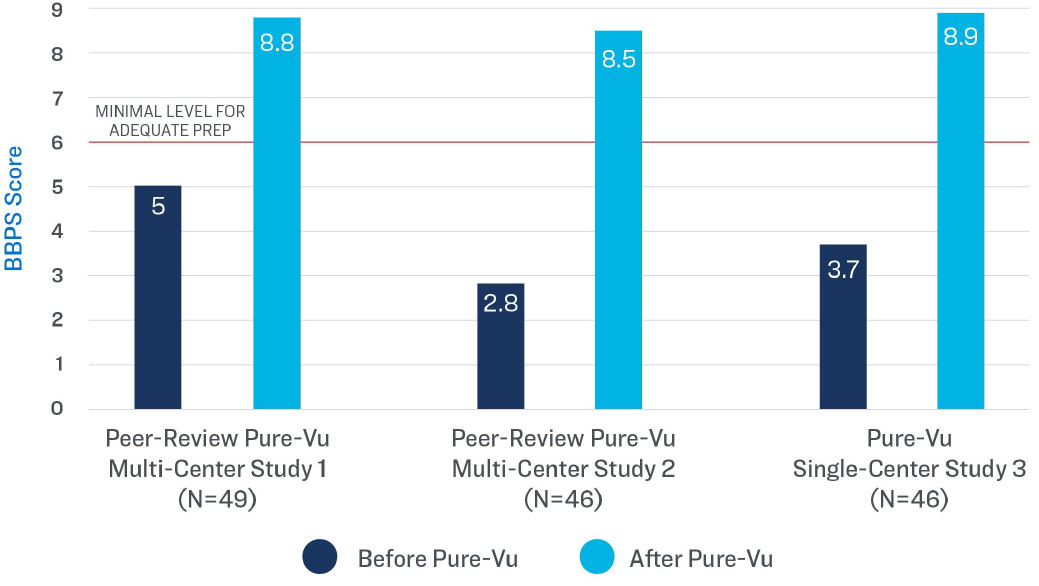
What data exists to support the clinical efficacy of the Pure-Vu® system?
In three multi-center studies and one single-center study, patients presenting with an inadequately prepped colon (as determined by the Boston Bowel Preparation Scale) became adequately prepped for colonoscopy after cleansing with the Pure-Vu® System.
BBPS Rating Before and After the
Use of the Pure-Vu® System
Technical Support
How many cases have been performed with the Pure-Vu® System?
To date, more than 600 procedures have been performed globally with the Pure-Vu® System.
Is there a risk of perforation with the Pure-Vu System?
Three perforations have been reported with the Pure-Vu® System to date. – In the first case, the patient was laparoscopically repaired and sent home within 12 hours. There have been no long-term complications reported. In the second case, the perforation may have occurred during attempted retroflexion, which is known to elevate such risks particularly in a narrow anatomy. In the third instance, the perforation was in an 80-year-old woman and occurred around the sigmoid flexure. These three instances are the only reported adverse events associated with use of the Pure-Vu® System worldwide.
Perforations are identified as precautions within the Pure-Vu® IFU and are consistent with the associated risk for a standard colonoscopy.
What is the difference between the Pure-Vu® standard oversleeve and the Pure-Vu® slim oversleeve?
| Feature | Pure-Vu® Standard OverSleeve |
Pure-Vu® Slim OverSleeve |
|---|---|---|
| Number of Pulsed Water and Air Irrigation Jets at Distal Tip | 4 | |
| Number of Suction Channels at Distal Tip | 2 | |
| Inner Diameter of Suction Channels at Distal Tip | 4.0 mm | |
| Outer Diameter of Distal Tip | 21 mm | |
| Length of Low Friction Hydrophylic Coating on Distal Oversleeve | 80 cm | |
| Colonoscope Compatibility (Insertion Tube Outer Diameter Range) | 12.8 mm - 13.7 mm | 11.7 mm – 13.3 mm |
| Outer Diameter of the Oversleeve | Varies throughout the oversleeve. The widest segment is 21mm | |
What is the difference between the cleansing airflow rates in various modes (High, Medium, Low)?
In cleansing mode, the Pure-Vu® System has an air flow of 0 cc in low mode, 1100 cc/min in medium, and 1350 cc/min in high mode; and the irrigation water flow has a maximum flow of 645 cc/min for all settings, but on average runs at 530 cc/min.
How much fluid is infused during a typical procedure?
The amount of fluid used to cleanse the colon can fluctuate based on the level of dirtiness. However, on average, procedures use ~1L of fluid.
Does the Pure-Vu® System interfere with any therapeutic procedures?
No. Colonoscope working channel remains patent so standard therapeutic procedure can be performed.
Can I still use CO2 with the Pure-Vu® System?
Yes. Use of the Pure-Vu System does not change your ability to insufflate with CO2. The majority of insufflation in a Pure-Vu procedure is CO2. The air emitted from the Pure-Vu device represents only a fraction of the gas in the lumen. Rapid absorption of CO2 should still take place during and after the procedure. I have not had physicians note discomfort or delay from the air in procedures.
If the colon reaches a certain pressure, does the machine stop administering air?
No. The internal and external sensors deliver a constant air flow. The internal airflow measured by the Pure-Vu® System is used to speed up or slow down the suction pumps.
Can the Pure-Vu® System be used during the colonoscopy when employing the water exchange and water immersion techniques?
Yes. Physicians have found benefit and success with utilizing the Pure-Vu® System during the water exchange and water immersion techniques. MOTUSGI is currently undergoing a single-center study to better assess the utility of our system in that application.
What resources are available to assist with System set-up and procedure support?
MOTUSGI is committed to assisting its customers before, during and after Pure-Vu® System procedures. In addition to a robust inservice program for physicians, nurses and technical staff, MOTUSGI provides hands-on support until customers are comfortable and proficient users of the System. Additionally, an intuitive Helpful Hints guide and instructive video are available for ongoing loading and unloading reference.
